Abstract
Introduction: Bruton's tyrosine kinase (BTK) is a key component of the B-cell receptor (BCR) signaling pathway. Targeting this pathway has proven highly effective in patients with chronic lymphocytic leukemia (CLL) and other B-cell malignancies. However, mutations in BTK develop with both covalent (cBTKi) and non-covalent inhibitors (ncBTKi) resulting in treatment resistance and disease progression. In addition, increased expression of the transcription factor IKZF3 may contribute to resistance to cBTKi/ncBTKi. Thus, novel therapeutic mechanisms are needed that target BCR signaling, particularly in patients whose disease has relapsed or is refractory (R/R) to existing BTK targeting therapies. NX-2127 is a novel small molecule that drives targeted BTK and IKZF3 degradation through ubiquitination and proteasomal degradation. This BTK degradation and immunomodulatory activity represents a novel mechanism of action and may overcome resistance to currently available novel agents including cBTKi and ncBTKi, addressing the unmet medical needs of patients whose disease is R/R to any BTKi (including ncBTKi) and a BCL2 inhibitor.
Methods: NX-2127-001 is a first-in-human, multicenter, US-based, open-label, Phase 1 dose-escalation (Phase 1a) and cohort-expansion (Phase 1b) trial, evaluating the safety, tolerability, and preliminary efficacy of NX-2127 in adult patients with R/R CLL and B-cell malignancies. Patients receive NX-2127 orally once daily in 28-day cycles starting at 100 mg. We report the initial findings from the Phase 1a portion of the trial and the rationale for initiating the Phase 1b portion at 100 mg for patients with CLL.
Results: As of 16 June 2022, 28 patients (17 CLL/SLL) were enrolled at dose levels 100, 200 and 300 mg. Patients were predominantly male (64.3%) with a median age of 76 (range 61-92) years. The most common adverse events in all patients are summarized in Table 1. One dose-limiting toxicity (DLT) of cognitive impairment was observed in a patient with CLL at 300 mg. No DLTs were observed in non-CLL indications.
As of 16 June 2022, 17 patients with CLL were enrolled having received a median of 6 prior therapies (range 2-12). All have previously received a BTKi and 76.5% had also received venetoclax. Poor prognostic factors include unmutated IGHV (23.5%), mutations/deletions in TP53 (17.6%). Of the 14 CLL patient samples tested, mutations included BTK [C481 (29%), L528 (29%), T474 (14%), V416 (7%)] and BCL2 (14%). Mutations C481, V416 and L528 result in loss of BTK kinase function.
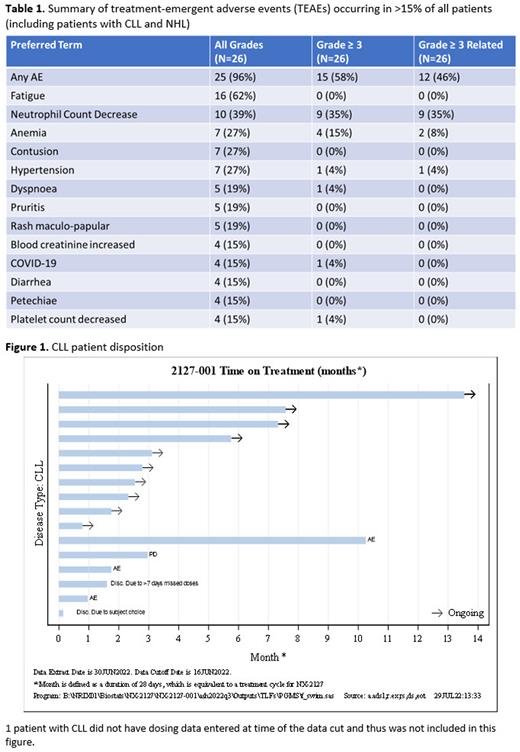
A mean BTK degradation of 86% was observed in all patients by Cycle 1 Day 22 with a mean degradation of 83% in patients with CLL, resulting in decreased BCR signaling as measured by reduction of plasma CCL4. Immunomodulatory activity, as evidenced by Ikaros (IKZF1) degradation, was observed at all dose levels and in all indications. 10 patients with CLL continue on study (Figure 1). There were 12 response-evaluable patients with CLL. The best overall response rate (ORR) was 33% with evidence that ORR increases with longer follow up (ORR: 16.7% at 2 mos, 42.9% at 4 mos, 50% at 6 mos). Importantly, responses were noted in BTKi/BCL2 double-refractory patients and those who progressed on a ncBTKi.
Conclusions: Double- and emerging triple-refractory CLL (patients who progressed on cBTKi, ncBTKi, and a BCL2 inhibitor) represents a major unmet medical need with no approved therapeutic options and poor survival. These patients may thus benefit from the interruption of BTK kinase-independent scaffolding signaling. In this first-in-human, first-in-class study of a BTK degrader, clinical responses and benefit were observed in heavily pretreated (median 6 prior therapies) patients with CLL who have poor prognostic factors, including those with BTK mutations resistant to cBTKi and ncBTKi, BCL2 mutations and those who were previously treated with both BTKi and BCL2 inhibitors. These data support further clinical development of NX-2127 in CLL, including expansion at the 100 mg dose level, and continued dose exploration for other B-cell malignancies. (NCT04830137).
Disclosures
Mato:Curio: Honoraria; AbbVie: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; Medscape: Honoraria; AstraZeneca: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; DTRM Biopharma: Honoraria, Research Funding; Octopharma: Honoraria, Research Funding; LOXO: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Dava: Honoraria; Pharmacyclics, LLC: Honoraria, Research Funding; Pfizer: Research Funding; TG Therapeutics, Inc: Honoraria, Research Funding; Nurix: Research Funding; Acerta: Research Funding; PER: Honoraria; BMS: Honoraria; Adaptive Biotechnologies: Honoraria; PerView: Honoraria. Wierda:Sanofi: Consultancy; Karyopharm: Research Funding; Miragen: Research Funding; Genzyme: Consultancy; Cyclacel: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Genentech: Research Funding; Juno: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Gilead Sciences: Research Funding; Xencor: Research Funding; Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; GSK/Novartis: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding. Ai:Secura Bio: Consultancy; More Health: Consultancy; Kymera: Consultancy; Kite: Consultancy; AC therapeutics: Consultancy; Acrotech: Consultancy; BeiGene: Consultancy; Walking Fish: Consultancy. Flinn:2seventy bio: Research Funding; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Incyte: Research Funding; Triphase Research & Development Corp: Research Funding; Century Therapeutics: Consultancy; Gilead Sciences: Research Funding; Genentech: Consultancy, Research Funding; Genmab: Consultancy; Kite Pharma: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; Hutchison MediPharma: Consultancy; CALGB: Research Funding; Loxo@Lilly: Research Funding; Curis: Research Funding; Infinity Pharmaceuticals: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Portola Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Trillium Therapeutics: Research Funding; BeiGene: Consultancy, Research Funding; Forty Seven: Research Funding; IGM Biosciences: Research Funding; Myeloid Therapeutics: Research Funding; CALIBR: Research Funding; Xencor: Consultancy; Secura Bio: Consultancy; Biopath: Research Funding; Bristol Myers Squibb: Research Funding; Pharmacyclics: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Seattle Genetics: Research Funding; Takeda: Consultancy; TG Therapeutics: Consultancy, Research Funding; Unum Therapeutics: Research Funding; Verastem: Consultancy, Research Funding; Acerta Pharma: Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; ArQule: Research Funding; Agios: Research Funding; Celgene: Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Tessa Therapeutics: Research Funding; Millenium Pharmaceuticals: Research Funding; Fate Therapeutics: Research Funding; Epizyme: Research Funding; CTI Biopharma: Research Funding; City of Hope National Medical Center: Research Funding; Forma Therapeutics: Research Funding; Servier Pharmaceuticals: Consultancy; TCR2 Therapeutics: Research Funding; Constellation Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Patel:Exelixis: Speakers Bureau; ION Pharma: Other: Leadership; Adaptive Biotechnologies: Honoraria; Bayer: Honoraria; Pharmacyclics: Honoraria; Blueprint Pharmaceuticals: Research Funding; Immunogen: Research Funding; Compugen: Research Funding; Accutar Biotech: Research Funding; Astellas: Research Funding; Adagene: Research Funding; Olema: Research Funding; Zymeworks: Research Funding; TeneoBio: Research Funding; Silicon Therapeutics: Research Funding; Samumed: Research Funding; Relay Therapeutics: Research Funding; Nurix: Research Funding; Novartis: Research Funding; NGM Biopharmaceuticals: Research Funding; Black Diamond Therapeutics: Research Funding; BioTheryX: Research Funding; Puretech: Research Funding; IgM Biosciences: Research Funding; MabSpace: Research Funding; Treadwell: Research Funding; Artios: Research Funding; ORIC: Research Funding; Vigeo: Research Funding; TopAlliance BioSciences Inc: Research Funding; Tesaro: Research Funding; Takeda: Research Funding; Taiho Pharmaceutical: Research Funding, Speakers Bureau; Syndax: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Ribon Therapeutics: Research Funding; Prelude Therapeutics: Research Funding; Portola Pharmaceuticals: Research Funding; Placon: Research Funding; Pfizer: Honoraria, Research Funding; Moderna Therapeutics: Research Funding; Mirati Therapeutics: Research Funding; Millennium: Research Funding; Merck: Research Funding; Macrogenics: Research Funding; Lycera: Research Funding; LSK Biopartners: Research Funding; Loxo: Research Funding; Kymab: Research Funding; Klus Pharma: Research Funding; Janssen: Honoraria, Research Funding; Jacobio: Research Funding; Incyte: Research Funding; Ignyta: Research Funding; Hutchison MediPharma: Research Funding; Hengrui Therapeutics: Research Funding; H3 Biomedicine: Research Funding; GlaxoSmithKline: Research Funding; Gilead Sciences: Research Funding; Genentech/Roche: Honoraria, Research Funding, Speakers Bureau; FORMA Therapeutics: Research Funding; Evelo Therapeutics: Research Funding; EMD Serono: Research Funding; Eli Lilly and Company: Research Funding; Daiichi Sankyo: Research Funding; Cyteir Therapeutics: Research Funding; Clovis Oncology: Research Funding; CicloMed: Research Funding; Checkpoint Therapeutics: Research Funding; Celgene: Research Funding, Speakers Bureau; Boehringer Ingelheim: Research Funding; BioNTech AG: Research Funding; AstraZeneca: Research Funding; Aileron Therapeutics: Research Funding; Agenus: Research Funding; ADC Therapeutics: Research Funding; Acerta Pharma: Research Funding; Pfizer/EMD Serono: Consultancy; Pharmacyclics/Janssen: Consultancy. Patel:Trillium Therapuetics/Pfizer: Consultancy, Research Funding; Fate Therapeutics: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; CRISPR Therapeutics: Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; MEI Pharma: Consultancy, Research Funding; Morphosys: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Epizyme: Consultancy, Research Funding; Caribou Biosciences: Consultancy; Curis, Inc: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Genetech/Roche: Consultancy, Research Funding, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; Nurix: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; Kite pharma: Consultancy, Research Funding, Speakers Bureau; Loxo Oncology: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Aptevo Therapeutics: Research Funding; ADC Therapeutics: Consultancy; Abbvie: Consultancy; Adaptive Biotechnologies: Research Funding; Velos Bio: Research Funding. O'Brien:AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, NOVA Resea: Consultancy; Acerta, Alliance, Beigene Ltd, Caribou Biosciences Inc, Gilead, Kite, Loxo Oncology, Mustang, Nurix Therapeutics Inc, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics.: Research Funding. Bond:Novartis: Research Funding; Nurix: Research Funding; Kite/Gilead: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria. Roeker:Qilu Puget Sound Biotherapeutics: Research Funding; Ascentage: Consultancy; Aptose Biosciences: Research Funding; Abbott Laboratories: Current equity holder in publicly-traded company; TG Therapeutics: Consultancy; Pfizer: Consultancy, Research Funding; Pharmacyclics: Consultancy; Loxo Oncology: Consultancy, Other: Travel support, Research Funding; Janssen: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months. Siddiqi:Astrazeneca: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Research Funding, Speakers Bureau; Jannsen: Speakers Bureau; Juno Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; BMS: Consultancy; Kite Pharma: Consultancy, Research Funding; TG Therapeutics: Research Funding; Oncternal: Research Funding; Ascentage Pharm: Research Funding. Wang:OncLive: Honoraria; Studio ER Congressi: Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Celgene: Research Funding; Genmab: Research Funding; Genentech: Consultancy, Research Funding; InnoCare: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria; Lilly: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Loxo Oncology: Research Funding; Molecular Templates: Research Funding; Vinverx: Research Funding; Dava Oncology: Honoraria; MJH Life Sciences: Honoraria; IDEOlogy Health: Honoraria; Eastern Virginia Medical School: Honoraria; Moffit Cancer Center: Honoraria; Practice Point Communications (PPC): Honoraria; Physicians Education Resources (PER): Honoraria; Pepromene Bio: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Merck: Honoraria; LLC TS Oncology: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; Oncology Specialty Group: Honoraria; Deciphera: Consultancy; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Acerta Pharma: Honoraria, Research Funding; AbbVie: Consultancy. Sun:Genmab: Research Funding. Abdel-Wahab:Envisagenics Inc., AIChemy, Harmonic Discovery Inc., and Pfizer Boulder: Membership on an entity's Board of Directors or advisory committees; H3B Biomedicine, LOXO Oncology, and Nurix Therapeutics: Research Funding; H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen: Consultancy. Schwab:Nurix Therapeutics, Inc.: Current Employment. Tan:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Meredith:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gessner:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kim:Nurix Therapeutics, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wiestner:Nurix: Research Funding; Merck: Research Funding; Abbvie company: Research Funding; Pharmacyclics: Research Funding; GenMab: Research Funding; Acerta Pharma: Research Funding; Verastem: Research Funding. Danilov:Incyte: Consultancy; Genentech: Consultancy; Morphosys: Consultancy; MEI: Consultancy, Research Funding; Takeda Oncology: Research Funding; Astra Zeneca: Consultancy, Research Funding; Beigene: Consultancy; Bristol-Meyers-Squibb: Consultancy, Research Funding; Bayer Oncology: Research Funding; Abbvie: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Pharmacyclics: Consultancy; Cyclacel: Research Funding; GSK: Consultancy.
OffLabel Disclosure:
NX-2127 is a novel small molecule that drives targeted BTK and IKZF3 degradation through ubiquitination and proteasomal degradation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal